In recent years, the family planning landscape has undergone a remarkable transformation. With easy access to effective contraception and evolving gender roles, more women are not just pursuing higher education and building fulfilling careers but also taking charge of their reproductive health [1]. This shift celebrates our individual, social, and economic progress, inspiring a new generation of women who can balance their aspirations with the desire for a family.
Understandably, many women choose to delay starting a family until they feel stable in their careers, have found a suitable partner or have established a particular lifestyle. This can lead them into their late 30s or even 40s before actively pursuing pregnancy. Yet, here lies the challenge—our biological clock ticks to a different rhythm.
In this article, we will uncover the hurdles faced by those who choose to delay motherhood, discover the biological factors that contribute to age-related infertility, and explore the practical strategies that can help synchronise our modern lifestyles with the dream of becoming a mother. Rest assured, methods are available to preserve fertility, including achieving a balanced lifestyle, managing stress, and avoiding toxins. Let’s embark on this enlightening journey together!
What happens to a woman’s fertility as she delays motherhood, and how does biology play a role in this?
A woman’s biological clock is the metaphorical term for the natural decline in fertility and the limited time window during which a woman can conceive a child naturally. It’s like a ticking clock that reminds us of the time we have left to have a baby. As we will discuss later in this article, a woman’s biological clock is determined by her finite egg reserve, which decreases in quantity and quality as she ages.
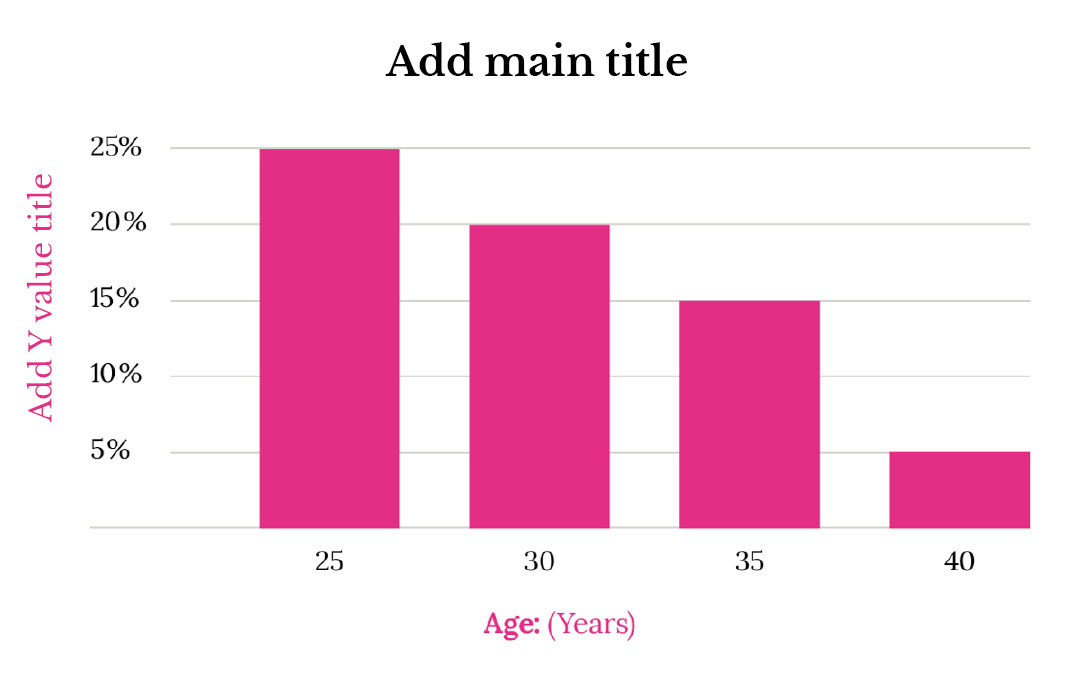
Studies on female fertility show a clear negative trend in a woman’s likelihood of getting pregnant throughout her lifetime (Figure 1). For instance, a woman in her 20s has a 20-25% chance of getting pregnant each menstrual cycle, while a woman in her mid-30s has only a 15% chance. Beyond 35, this decline becomes more rapid [1]. Consequently, the chances of conception decrease over our lifetime, and the journey towards motherhood can become a steeper climb.
By delving into the science behind age-related infertility, we can tackle this issue head-on and discover innovative methods that extend our fertility timelines. Armed with knowledge and understanding, time constraints shouldn’t mar the journey towards motherhood but should be approached with hope and determination. Let’s unravel the mysteries of egg ageing and unlock the possibilities.
The Biological Constraints: The Diminishing Oocyte Reserve
The key to age-related infertility lies in our oocytes, or what we commonly call eggs. These precious cells play a vital role in our fertility. Unfortunately, our eggs undergo a progressive ageing process that significantly impacts our chances of successful conception. The quantity and quality of our eggs decline, paving the way for hardships in starting a family.
(1) Decline in Egg Quantity
Women are born with all the eggs they will ever have, encapsulated in tiny fluid-filled sacs called follicles located in the ovaries. Within these follicles, the eggs grow and develop. During our time in the womb, our ovaries work overtime, making approximately 7 million follicles by the time we are only 20 weeks gestation [2]. After this point, women cannot produce new eggs; in fact, there is a continuous decline in the total number of eggs each month [3]. Consequently, only around 300,000-400,000 eggs remain when a girl enters puberty and will deplete to virtually none by the time a woman reaches menopause.
It’s natural for a woman to lose about 1,021 follicles each month during her reproductive years, which can accelerate as she ages. As a result, only about 300-500 follicles will develop enough to release a mature egg that can be ovulated and fertilised. It may seem like too many eggs are going to waste, but this high rate of follicle death is necessary for normal ovarian function. It helps select against “bad” eggs, increasing the chances that follicles containing healthy eggs will ovulate and develop into a healthy baby.
This natural decline in available eggs is a natural part of ageing. It is commonly referred to as diminished ovarian reserve. Ovarian reserve is an essential factor when it comes to fertility and may affect the chances of conception, leading to a longer time to pregnancy, some difficulties in achieving pregnancy naturally and a reduced response to fertility treatments like in vitro fertilisation (IVF). However, it’s essential to keep in mind that many factors can influence fertility, and there are various options available for those who may be experiencing diminished ovarian reserve. It’s also worth noting that there are many cases where individuals with this condition still go on to have successful pregnancies. It’s always best to speak with a healthcare provider who can provide guidance and support for those who may be concerned about their fertility.
(2) Decline in Oocyte Quality
Along with decreasing in number, the quality of our eggs begins to deteriorate as we age, in a process called “egg ageing.” This is a natural part of the ageing process and can lead to difficulties getting pregnant. While our egg reserve naturally plays a role in our ability to fall pregnant, most challenges as we age are due to declining egg quality. After all, having one perfectly formed egg is only necessary to have a healthy baby.
Birth rates for patients aged 35 who undergo IVF are a decent 32% per embryo transferred. However, for patients over 43 who use their eggs for IVF, these rates drop to below 5%. On the other hand, when young donor eggs are used instead of a woman’s eggs, birth rates remain above 30% for all ages. This stark difference highlights the importance of egg quality, which is closely tied to our biological clock. Therefore, working with our biological clock rather than against it may lead to a more natural and less stressful journey towards parenthood.
An egg is very different from any other cell in your body. It is a highly complex and specialised cell that plays a crucial role in reproduction. It contains various organelles (specialised structures within cells that perform specific functions), genetic material, protective layers, and essential components for successful reproduction. Unfortunately, as we age, the wear and tear of life causes many of these cellular components to deteriorate, disrupting the processes necessary for an egg to be fertilised and successfully develop into a viable embryo. As a result, fertility problems may arise, such as a low fertilisation rate, poor embryonic development, and a higher risk of spontaneous miscarriage and chromosomal abnormalities.
Although the ageing process of oocytes is complicated and still not completely understood, it is now widely acknowledged that mitochondrial dysfunction plays a significant role in age-related infertility [2].
Mitochondria are like tiny powerhouses inside our cells. Imagine them as small, little energy factories that work tirelessly to keep our bodies running smoothly. These tiny powerhouses are crucial in producing energy for our cells, much like a factory. They utilise nutrients from our food to create ATP molecules, the molecular energy currency behind all biological functions: for example, moving your muscles, thinking, and breathing. In other words, mitochondria are responsible for generating the energy that enables us to perform everyday activities. To give you an idea of ATP’s life-sustaining importance, your body converts a volume of ATP equal to your entire weight daily.
In the context of our oocytes, they play a vital role in generating enough energy to power the complex processes involved in oocyte development and maturation. Hence, the quality of an oocyte is dependent on proper mitochondrial function. This is emphasised by its abundance, with an estimated 10,000 mitochondria in a fully mature oocyte, over ten times the amount found in other cells in the human body. Unfortunately, a byproduct of this energy production is producing reactive oxygen species (ROS). ROS are highly reactive molecules that can cause damage to various cellular components, including DNA. Typically, oocytes have an antioxidant defence system to neutralise the harmful effects of ROS and protect against DNA damage. Mitochondria are, in fact, our oocyte’s first line of defence against ROS, having specialised enzymes and molecules that act as antioxidants to counteract the damaging effects of ROS. However, mitochondrial dysfunction can disrupt this system, leading to an imbalance between ROS production and their removal. This imbalance, commonly referred to as oxidative stress, increases ROS levels in the oocytes. Raised ROS also damage mitochondria themselves. Consequently, a vicious cycle between oxidative stress and mitochondrial dysfunction ensues, continually deteriorating oocyte quality.
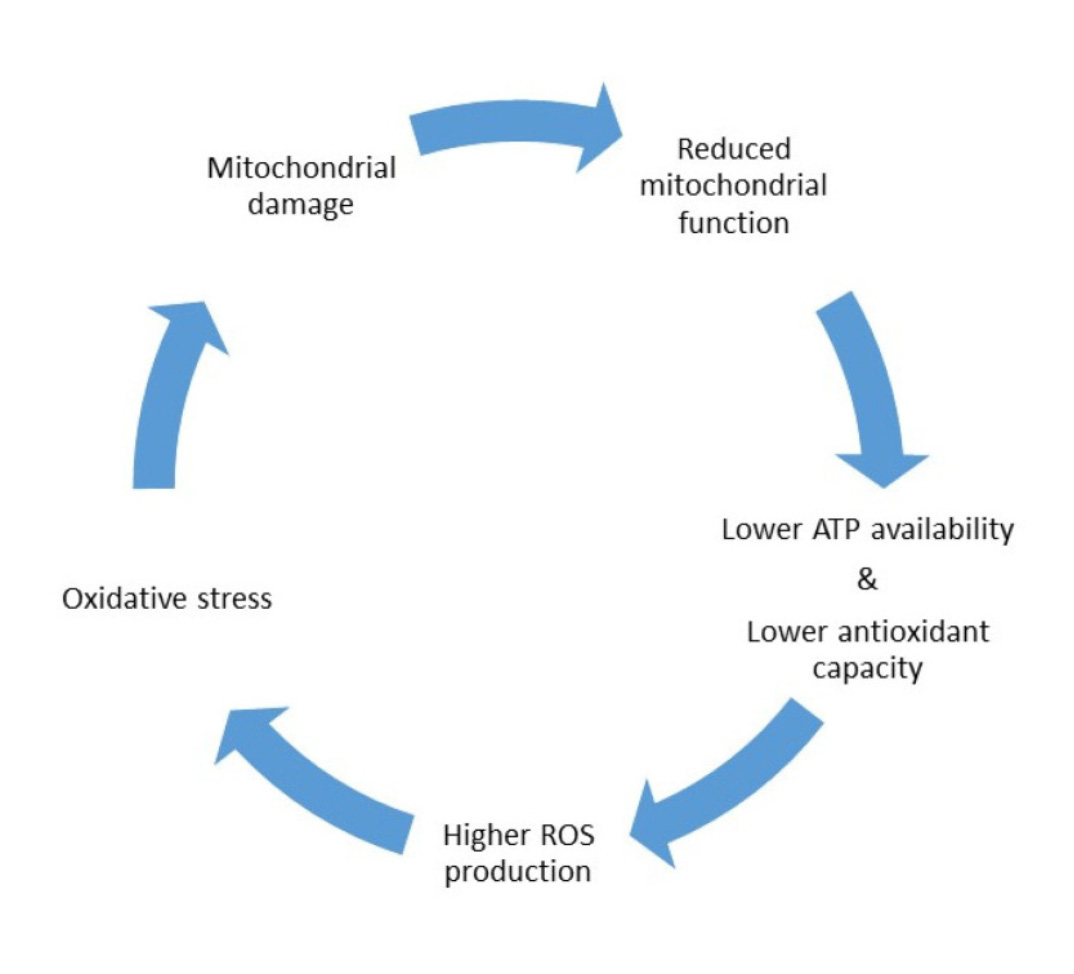
Unfortunately, as we age, the number and quality of mitochondria in our oocytes begin to decline. The natural wear and tear over time leads to mitochondria accumulating damage and becoming less efficient in producing energy and defending against ROS.
There are two significant consequences of the lack of energy and increased oxidative stress that come with mitochondrial dysfunction:
(1) The accumulation of DNA damage: Over time, the DNA within the oocytes may experience various types of damage, such as mutations or breaks, which can impair the normal functioning of the cells [4]. These damaged oocytes have a reduced capacity to support fertilisation and development, which can result in fertility issues or an increased risk of miscarriage.
(2) The presence of chromosomal abnormalities: As women age, their oocytes are more likely to have an incorrect number of chromosomes, a condition called aneuploidy. This occurs when the chromosomes do not segment properly during oocyte maturation or when older oocytes are retained in the ovary for extended periods, allowing more time for alterations. These chromosomal abnormalities can lead to failed fertilisation, early pregnancy loss, or genetic disorders in offspring. Clinically, this manifests as a low fertilisation rate, poor embryonic development, and an increased risk of spontaneous miscarriage and chromosomal abnormalities [5].
Therefore, as we age, the quality and quantity of a woman’s eggs naturally decline. It is consequently better for women to have children during their reproductive prime when they are more likely to succeed from an evolutionary perspective. However, the development of Assisted Reproductive Techniques (ART) such as in vitro fertilisation (IVF) and elective egg freezing has extended some women’s capacity to start a family beyond their biological clock [6] [7]. This has been a positive development for numerous women of advanced age, empowering those who may have previously faced limitations due to biological factors.
It’s important to remember that although these methods have revolutionised the world of fertility, they cannot guarantee success and can only combat the natural decline in fertility with age to a certain extent. The effectiveness of ART can vary based on many factors, including the woman and her partner’s age, overall health, and specific fertility issues. Moreover, these techniques can be expensive, time-consuming, emotionally draining, and come with risks such as multiple pregnancies and potential complications for both the mother and the baby. Therefore, relying entirely on assisted techniques to combat age-related infertility is not advisable, and all women should take active steps to help safeguard their egg supply.
Methods to Safeguard Your Oocyte Quality
Whether you are attempting to fall pregnant shortly, planning to freeze your eggs, undergo fertility treatment, or want to enhance your well-being, preserving your ovarian reserve should be something every woman of every age should do. While there are no guaranteed ways to protect female oocytes indefinitely, several natural interventions may help support oocyte health and extend their lifespan to some extent.
Achieving a balanced lifestyle
Age is commonly believed to be the only factor affecting women’s oocyte reserve. However, several other environmental factors can impact the quality of oocytes, such as diet [8], weight [9], diseases, stress [10], exercise [11], and exposure to toxins [7]. Taking a holistic approach to your reproductive health is crucial to protect your oocytes as you age. This involves leading a healthy, balanced lifestyle that nourishes your body and preserves fertility. While certain supplements may enhance oocyte quality, it is essential to remember that they are not a magic solution. Therefore, before taking supplements, it is vital to lay the foundations for a fruitful future by adhering to the following fundamental principles:
- Prioritise Nutrition: Opt for nutrient-dense foods rich in antioxidants, vitamins, and minerals. A diet with plenty of fruits, vegetables, whole grains, lean proteins, and healthy fats can help support optimal oocyte quality. As discussed previously, avoiding processed foods and instead focusing on a Mediterranean Diet is the best nutritional approach to boosting fertility. In one study, adherence to a Mediterranean diet increased the probability of couples achieving pregnancy after IVF/ICSI treatment by 40% [12].
- Managing Blood Sugar Levels: High blood sugar levels can harm female fertility by causing ovulatory dysfunction, disrupting hormone balance, and affecting egg quality [13]. This can also lead to complications during pregnancy. Maintaining healthy blood sugar levels through a balanced diet, regular exercise, and medical supervision is crucial for optimising the chances of conceiving and maintaining a healthy pregnancy.
- Get Moving: Regular physical exercise is essential for overall well-being and can boost ovarian function. However, avoid overdoing it, as overexercising can put too much stress on the female body and dampen fertility [11]. Engage in moderate exercise, such as brisk walking, weight lifting, swimming, or yoga, to improve blood flow, manage stress, and promote fertility.
- Manage Stress Levels: Chronic stress, including oocyte quality, can negatively impact female reproductive health [10]. Explore stress reduction techniques such as meditation, acupuncture, deep breathing exercises, or engaging in hobbies to maintain a calm and balanced state of mind.
- Adequate Sleep: Aim for 7-8 hours of sleep each night. Sufficient rest facilitates hormone regulation, reduces inflammation, and supports reproductive health [14].
- Avoid Toxins: Minimise exposure to environmental pollutants, chemicals, and toxins that can harm reproductive health [15]. Opt for organic, pesticide-free foods, choose non-toxic personal care and household products, and consider reducing alcohol and caffeine consumption.
Supplementation
Supplements have gained popularity as potential allies in the quest for improved egg quality. While maintaining a healthy lifestyle should remain paramount, these supplements may further boost reproductive health by increasing your oocytes’ mitochondria and antioxidant defence systems (see the “science explained” section above for more details). Although each of the below supplements requires an entire article in its own right, here is a brief overview of some scientifically backed options to consider.
Coenzyme Q10
Coenzyme Q10 is a powerful antioxidant that plays a crucial role in cellular energy production. It has been shown to benefit oocyte quality by reducing chromosomal abnormalities, enhancing mitochondrial function, combating oxidative stress, and protecting the eggs from damage [16]. By enhancing energy production within the cells, coenzyme Q10 can help support healthy egg development and maturation, potentially improving fertility outcomes. Supplementing with coenzyme Q10 may benefit women seeking to optimise their reproductive health and increase their chances of successful conception [17].
Melatonin
Melatonin is a powerful antioxidant and hormone that regulates the sleep-wake cycle. Research suggests that melatonin can benefit oocyte quality by reducing oxidative stress, protecting the eggs from damage, and supporting healthy mitochondrial function. By promoting a more favourable environment for egg development and maturation, melatonin can improve fertility outcomes in women. Supplementing with melatonin has been shown to enhance oocyte quality. It may be particularly beneficial for women experiencing age-related decline in fertility [18].
L-Carnitine
L-carnitine is an amino acid key in energy production and metabolism within cells, including oocytes. It facilitates the transport of fatty acids into mitochondria, thereby supporting mitochondrial function and energy production [19]. By enhancing energy metabolism and reducing oxidative stress, L-carnitine can improve oocyte quality and contribute to better reproductive outcomes. Research suggests that supplementation with L-carnitine may enhance oocyte maturation, fertilisation, and embryo quality in women undergoing assisted reproductive technologies [20].
Myo-inositol & D-Chiro-Inositol
Myo-inositol and D-chiro-inositol are two essential forms of inositol that play crucial roles in cellular signalling, insulin sensitivity, and ovarian function [21]. They are involved in various pathways related to oocyte quality, including cell growth, hormone regulation, and glucose metabolism. Research has shown that the correct ratio and supplementation of Myo-inositol and D-Chiro-inositol can help improve oocyte quality by supporting proper follicle development, hormone balance, and overall reproductive function [22] [23]. This supplementation has been linked to enhanced egg quality, increased ovulation rates, and improved outcomes in women with conditions like polycystic ovary syndrome (PCOS) and infertility [24].
Omega-3 Fatty Acids
Omega-3 fatty acids are essential nutrients associated with various health benefits, including improved fertility and reproductive function. These fatty acids are vital in reducing inflammation, promoting healthy cell membrane function, and supporting overall reproductive health. Research suggests that omega-3 fatty acids can positively impact oocyte quality by enhancing mitochondrial function, reducing oxidative stress, and supporting optimal follicle development [25]. By incorporating omega-3 fatty acids into the diet through sources like fish, flaxseeds, and walnuts, individuals may experience improved oocyte quality, potentially leading to better fertility outcomes [26].
Vitamin D
Vitamin D is crucial in reproductive health and has been linked to improved oocyte quality. Adequate vitamin D levels have been associated with enhanced ovarian function, hormonal balance, and follicular development [27] [28]. Vitamin D also helps regulate calcium levels within oocytes, which are essential for various cellular processes. Research suggests that optimising vitamin D levels through supplementation or sunlight exposure can improve oocyte quality, increasing the chances of successful fertility outcomes [28].
Folic Acid
Folic acid, also known as folate, plays a crucial role in DNA synthesis and repair, making it essential for cell division and development. In the context of oocyte quality, folic acid helps to maintain genetic stability and integrity, which is vital for producing healthy eggs [29]. Adequate levels of folic acid can also reduce the risk of chromosomal abnormalities in oocytes, thereby enhancing their quality and potentially improving the chances of successful fertilisation and embryo development. Incorporating folic acid into one’s diet or supplementation regimen can promote optimal oocyte quality and reproductive health [30].
DHEA
DHEA (dehydroepiandrosterone) is a hormone produced by the adrenal glands that has been found to potentially improve oocyte (egg) quality in women undergoing fertility treatment [31]. DHEA is believed to enhance the ovarian environment by increasing androgen levels, which may positively impact oocyte maturation and development [32]. Studies suggest that DHEA supplementation may help to improve egg quality [33] , increase pregnancy rates [32], and reduce the risk of chromosomal abnormalities in embryos [34].
Remember, supplementation should only be considered an adjunct to a healthy lifestyle. While scientific evidence supports certain supplements like CoQ10, L-Carnitine, omega-3 fatty acids, myo-inositol, D-chiro-inositol, and vitamin D for improving oocyte quality, consulting with a healthcare professional for personalised recommendations is essential. They can guide you on the appropriate dosages, durations, and combinations tailored to your needs.
Conclusion
In conclusion, age-related infertility poses a significant challenge for many women due to the decline in egg quality and quantity as they get older. Implementing a healthy lifestyle, including maintaining a balanced diet, regular exercise, and avoiding harmful habits like smoking, can help safeguard the oocyte reserve and optimise fertility potential. Additionally, supplementation with specific nutrients like antioxidants, Coenzyme Q10, and folate may offer further support in protecting egg health. By taking proactive steps to support overall health and egg quality, women can increase their chances of achieving successful conception and healthy pregnancy outcomes, even as they age.
References
- Delbaere, I., S. Verbiest, and T. Tydén, Knowledge about the impact of age on fertility: a brief review. Ups J Med Sci., 2020.
- Chiang, J., L. , et al., Mitochondria in Ovarian Aging and Reproductive Longevity. Ageing Res Rev., 2020.
- Baker, T., G., A QUANTITATIVE AND CYTOLOGICAL STUDY OF GERM CELLS IN HUMAN OVARIES. Proc R Soc Lond B Biol Sci., 1963.
- Van der Reest, J., et al., Mitochondria: Their relevance during oocyte ageing. Ageing Res Rev., 2021.
- Ávila, J., et al., Oxidative Stress in Granulosa-Lutein Cells From In Vitro Fertilization Patients. Reprod Sci., 2016.
- Harrison, B., J. , et al., Advanced maternal age: ethical and medical considerations for assisted reproductive technology. 2017.
- Varlas, V., N. , et al., Social Freezing: Pressing Pause on Fertility. 2021.
- Łakoma, K., O. Kukharuk, and D. Śliż, The Influence of Metabolic Factors and Diet on Fertility. Nutrients., 2023.
- Broughton, D., E. and K. Moley, H., Obesity and female infertility: potential mediators of obesity’s impact. Fertility and Sterility, 2017.
- Ilacqua, A., et al., Lifestyle and fertility: the influence of stress and quality of life on male fertility. Reprod Bio Endocrinol, 2018.
- Shufelt, C., L., T. Torbati, and E. Dutra, Hypothalamic Amenorrhea and the Long-Term Health Consequences. Semin Reprod Med., 2017.
- Vujkovic, M., et al., The preconception Mediterranean dietary pattern in couples undergoing in vitro fertilization/intracytoplasmic sperm injection treatment increases the chance of pregnancy. Fertility and Sterility, 2010.
- Faghfoori, Z., et al., Nutritional management in women with polycystic ovary syndrome: A review study. Diabetes Metab Syndr., 2017.
- Kloss, J., D. , et al., Sleep, sleep disturbance, and fertility in women. Sleep Med Rev, 2015.
- Mima, M., D. Greenwald, and S. Ohlander, Environmental Toxins and Male Fertility. Currl Urol Rep., 2018.
- Brown, A., M. and H. McCarthy, E., The Effect of CoQ10 supplementation on ART treatment and oocyte quality in older women. Human Fertility, 2023.
- Xu, Y., et al., Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: a randomized controlled trial. Reprod Bio Endocrinol, 2018.
- Reiter, R., J., et al., Aging-Related Ovarian Failure and Infertility: Melatonin to the Rescue. Antioxidants (Basel). 2023.
- Agarwal, A., P. Sengupta, and D. Durairajanayagam, Role of L-carnitine in female infertility. Reprod Bio Endocrinol, 2018.
- Li, J., et al., Biological roles of l-carnitine in oocyte and early embryo development. Mol Reprod Dev., 2021.
- Vitale, S.G., et al., How to Achieve High-Quality Oocytes? The Key Role of Myo-Inositol and Melatonin. Int J Endocrinol., 2016.
- Dinicola, S., et al., The rationale of the myo-inositol and D-chiro-inositol combined treatment for polycystic ovary syndrome. 2014.
- Kamenov, J. and A. Gateva, Inositols in PCOS. Molecules., 2020.
- Regidor, P.-A., et al., Management of women with PCOS using myo-inositol and folic acid. New clinical data and review of the literature. 2018.
- Abodi, M., et al., Omega-3 fatty acids dietary intake for oocyte quality in women undergoing assisted reproductive techniques: A systematic review. Eur J Obstet Gynecol Reprod Biol, 2022.
- Chiu, Y.-H., et al., Serum omega-3 fatty acids and treatment outcomes among women undergoing assisted reproduction. Hum Reprod., 2018.
- Xu, J., et al., Vitamin D3 Regulates Follicular Development and Intrafollicular Vitamin D Biosynthesis and Signaling in the Primate Ovary. Front Physiol., 2018.
- Fichera, M., et al., Vitamin D, reproductive disorders and assisted reproduction: evidences and perspectives. Int J Food Sci Nutr., 2020.
- Saini, S., et al., Folate supplementation during oocyte maturation positively impacts the folate-methionine metabolism in pre-implantation embryos. Theriogenology, 2022.
- Gaskins, A., J., et al., Dietary Folate and Reproductive Success Among Women Undergoing Assisted Reproduction. 2015.
- Sömmezer, M., et al., Dehydroepiandrosterone supplementation improves ovarian response and cycle outcome in poor responders. Reprod Biomed Online., 2009.
- HE., N., et al., Androgens (dehydroepiandrosterone or testosterone) for women undergoing assisted reproduction. Cochrane Database Syst Rev., 2015.
- Li CJ, Lin LT, and T. KH., Dehydroepiandrosterone Shifts Energy Metabolism to Increase Mitochondrial Biogenesis in Female Fertility with Advancing Age. Nutrients., 2021.
- Gleicher, N., A. Weghofer, and D. Barad, H., Dehydroepiandrosterone (DHEA) reduces embryo aneuploidy: direct evidence from preimplantation genetic screening (PGS). Reprod Bio Endocrinol, 2010.